The general objective of the project is to generate new methods and tools allowing the exploitation of the very large amount of available heterogeneous data (mostly imaging, and also contextual and biological data) for toxicity and tumor recurrence prediction within the context of prostate cancer radiotherapy. “Radiomics” is a concept in which high-throughput extraction of quantitative imaging features is used aimed at creating mineable databases from radiological images, which might reveal quantitative predictive or prognostic associations between images and medical outcomes. Such an approach could also allow improving the predictive models of both tumor response and organs-at-risk (OAR) toxicity. The final objective is to better adapt radiotherapy for individuals suffering from prostate cancer, by fully taking advantage of the most recent irradiation techniques, such as intensity modulated and guided adaptive radiotherapy (IMRT, ART, IGRT).
A large amount of heterogeneous data from 2500 prospective patients coming from different observation modalities will be integrated: i) Image data such as computed tomography (CT), cone beam CT (CBCT), MRI and PET; ii) patient clinical history, presented toxicity and recurrence events; iii) Therapy records, such as dosimetric data (dose volume histogram and 3D dose distribution) and iv) biological markers of radio-induced lethality (apoptosis, genetic polymorphism) and inflammation. Hence, by integrating all the available data, the aim of the project is the establishment of new statistical predictive models of digestive and urinary toxicities and recurrence following prostate cancer radiotherapy.
The major breakthrough of the present project is the original integration of dosimetric/imaging and biological data within the models, which is currently rare in the literature, especially for large cohorts. The complex urinary toxicity will be particularly explored. The design of these models will also allow identifying highly sensitive sub-volumes within the whole urinary and digestive structures, as well as dominant intraprostatic tumor, and could be used for tailoring of personalized adaptive radiotherapy treatments . Personalized treatments are expected to have a strong impact on patients outcome by significantly decreasing side effects and simultaneously increasing curability following prostate cancer radiotherapy.
Geniturinary Toxicity after Prostate Radiotherapy: Developement and validation of predictive models
Research groups around the world have attempted to study the potential of treatment optimization and individualization through the use of knowledge of the associations of treatment, clinical and dose factors with specific treatment-related dysfunctions. It is generally acknowledged in these studies that radio-induced side-effects are associated with a large number of factors that differ for each individual patient, with a great difficulty of inferring these complex models directly from current radiobiological knowledge.
In particular predictive models of urinary toxicity are commonly based on DVH of the whole bladder which reduces the 3D dose distribution within the organ to a unidimensional and discrete representation of the dose-volume relationship. Several limitations arise when only scalar values or DVH are used within predictive models: i) A DVH is limited to a single organ, ii) 3D dose distributions may lead to the same DVH, iii) the information on the spatial distribution of dose is lost by merely considering the organ volume thus ignoring the local variations and the potentially heterogeneous intra-organ radiosensitivity, iv) correlation may exist between adjacent DVH bins and v) more broadly, toxicity is a complex multiparametric phenomenon that may involve structures at different scales, from sub-organ parcels to large structures or regions whose response may additionally depend on individual radiosensitivity. These factors may explain the limited prediction capability of DVH-based models.
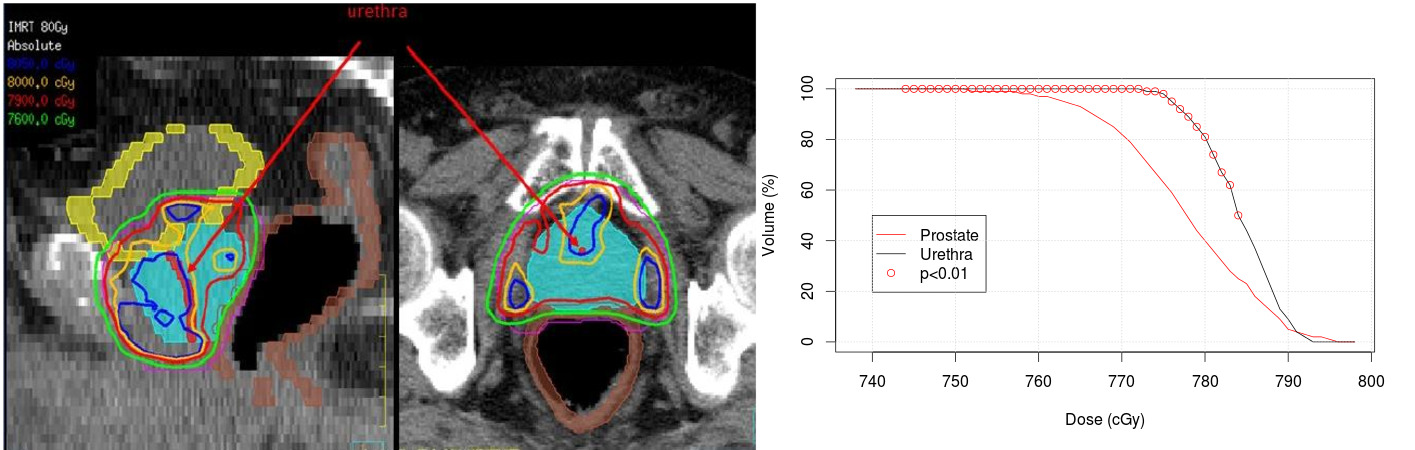
To date, all the urinary toxicity studies after EBRT have been focused on the dose to the bladder or parts of it, while the potential involvement of urethra damage remains largely unexplored due to inherent difficulty to identify this structure on the planning computed tomography (CT) scan. Indeed, the prostatic urethra has similar physical properties with its surrounding prostatic tissue, and as a result, this structure is completely invisible on the CT image.
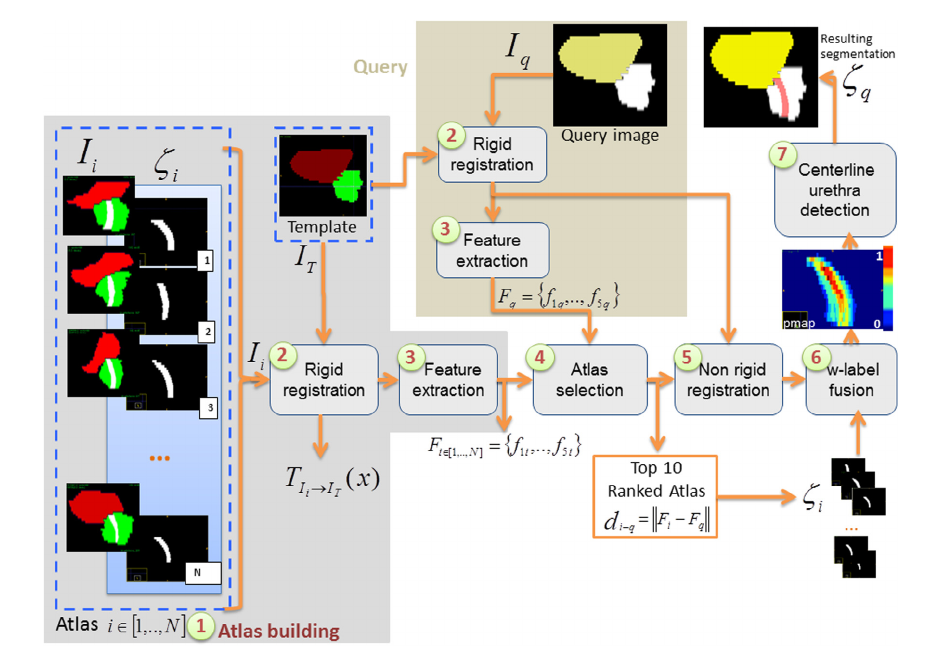
To assess the contribution of urethra damage to urinary toxicity, we developed and validated a weighted Multi-Atlas-Based Urethra Segmentation (MABUS) method which allows to accurately identify the urethra on planning CT images. In Figure 1, it is depicted the proposed framework of MABUS method. The atlas consists of CT images of brachytherapy-treated patients where the urethra is visible thanks to the use of urinary probe. For a new patient, geometric features are extracted from the image to be segmented and compared to the features from the atlas database. The urethra for this new patient is, then, defined by combining the urethras of the most similar atlases in a weighed-fusion process.
The proposed segmentation method was applied to 95 patients treated with IMRT. The dose within the urethra was assessed from the 3D planning dose distribution and compared to the dose to the prostate. A comparison between the prostate and urethra DVHs was performed using a Wilcoxon non parametric test. Urethra and prostate DVHs were significantly different. The bin-wise DVH comparison shows that the volume (%) receiving a dose between D74 Gy and D79 Gy by the urethra was significantly higher than in the prostate (Figure 2).
The availability of individual, 3-dimensional, dosimetric information permits the quantitative assessment of dose-volume relations for specific urinary endpoints by investigating the correlations between dose-volume data and toxicity data. Thus, an analysis of the dose distribution in the bladder and the urethra at lower spatial scales, could potentially reveal a more local dose-effect relationship. Additionally, by linking toxicity data to considered clinical and dosimetric factors, the underlying relationships can be understood and translated into recommendations for treatment planning in clinical practice. Different approaches can be used to geometrically represent the 3D dose distribution in a single coordinate system via a spatial normalisation for a joint analysis of dose at the lowest sampling scale (pixel and voxel levels). Either by building a parametric mapping via an intermediate spherical /cylindrical coordinate system such as the Dose Surface Maps (DSM) in 2D , in 3D or via 3D anatomical non-rigid registration, herein called 3D Dose Volume Maps (DVM). The objective of such analyses is to identify anatomical regions correlated with toxicity that are potentially more predictive than the dose to the whole bladder.
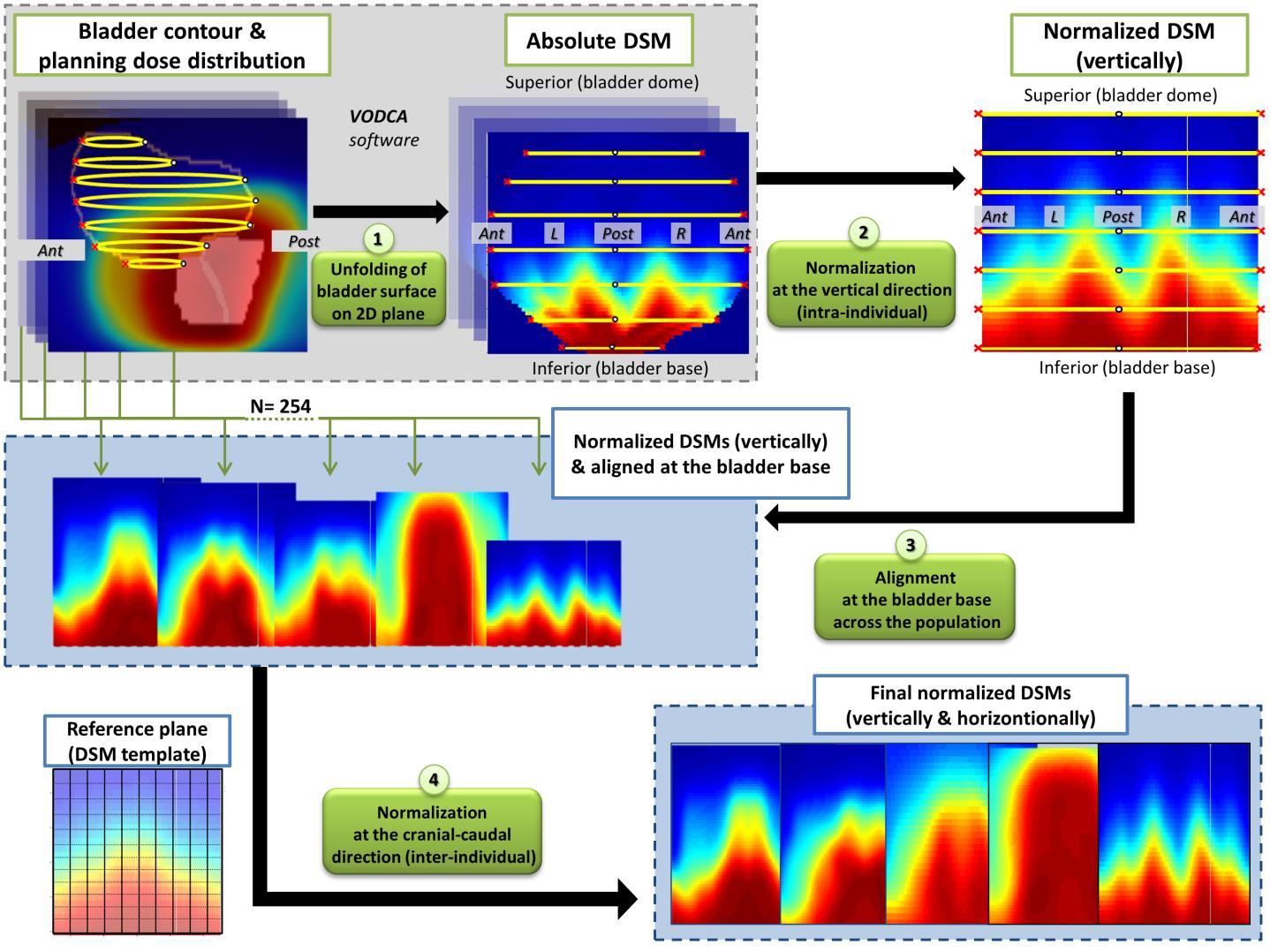
First, a dose-surface map (DSM) analysis of the dose to the surface of the bladder was performed to explore the local dose-response relationship for different urinary symptoms. DSMs were generated by virtually unfolding bladder surfaces in a 2D plane, as shown in Figure 3, followed by pixel-by-pixel comparisons between patients with and without toxicities. The results were compared with previous DSM studies in order to evaluate the impact of different cohorts in the identification of radiosensitive subregions.
Three sub-surfaces were identified in the bladder: posterior for acute retention (AUC =0.70), posterior-superior for late retention (AUC =0.72), and inferior-anterior and lateral for late dysuria (AUC=0.73). Average dose differences between patients with/without toxicity ranged from 7.9 Gy to 10.9 Gy. Among five symptoms comparable between cohorts, Ssurf in common was found only for late dysuria, with a good spatial agreement.
The next step was to analyze the entire 3D planning dose distribution in the bladder and the urethra without making any prior assumptions regarding the location of regions at risk. A sophisticated approach based on 3D Dose volume map (DVM) analysis was employed and the local dose-effect relationship was evaluated at voxel level. The different pre-processing, including: i) an accurate spatial normalization to a single coordinate system, ii) the mapping of the doses to be analyzed and, iii) a reliable methodology to perform local statistical analysis, were thoroughly validated. The workflow is described in Figure 4.
Five sub-volumes were identified: one in the urethra for acute incontinence and four in the bladder (posterior-inferior for acute retention, posterior for late retention and late dysuria and superior for late hematuria). Dose differences ranged from 1.2 Gy to 9.3 Gy and the prediction capabilities (AUC) ranged from 0.71 to 0.82. The subregions for acute incontinence, late retention and late dysuria were confirmed in a large external population (RADAR trial).
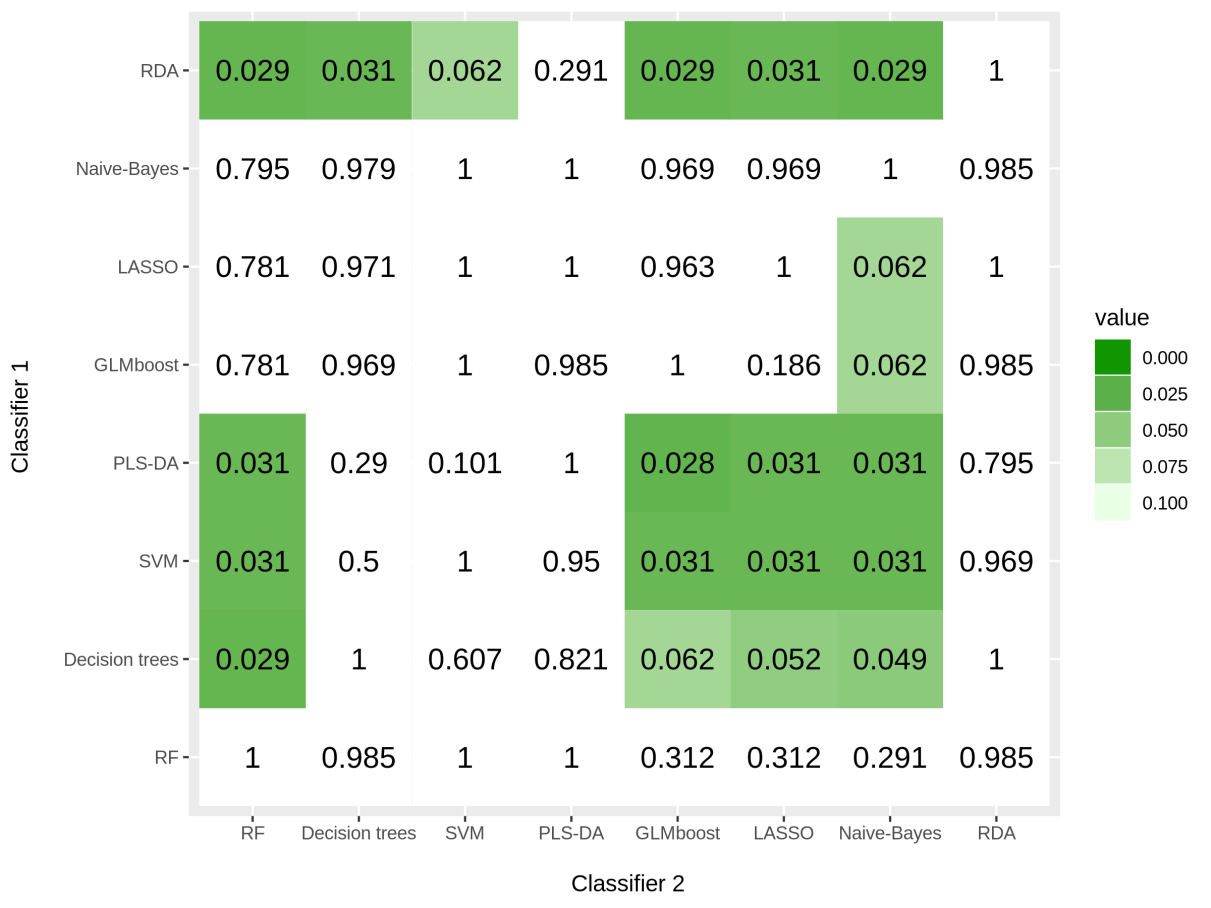
The vast majority of existing studies are based upon traditional regression approaches (e.g. logistic or Cox regression) to identify the most important predictors. Although these approaches are often preferred over machine learning techniques due to their interpretability, the prediction power of such models may be modest. Machine learning techniques can potentially increase toxicity prediction as they rely on previous informative examples. Plethora of methods are emerging without a clear advantage of its use in this context as they application is not straightforward. For this purpose, we compared several machine-learning strategies together with different minority class oversampling techniques for prediction of urinary toxicity following prostate cancer radiotherapy using dosimetric and clinical data. The performance of these classifiers was evaluated on the original dataset and using four different synthetic oversampling techniques. Results suggest that, regardless of the technique, oversampling always increases the prediction performance of the models (p=0.004). Overall, oversampling with Synthetic Minority Oversampling Technique (SMOTE) followed by Edited Nearest Neighbour algorithm (ENN) together with Regularized Discriminant Analysis (RDA) classifier provide the best performance (AUC=0.71).
Rectal bleeding prediction following prostate cancer radiotherapy using Machine Learning and Deep Learning Approaches
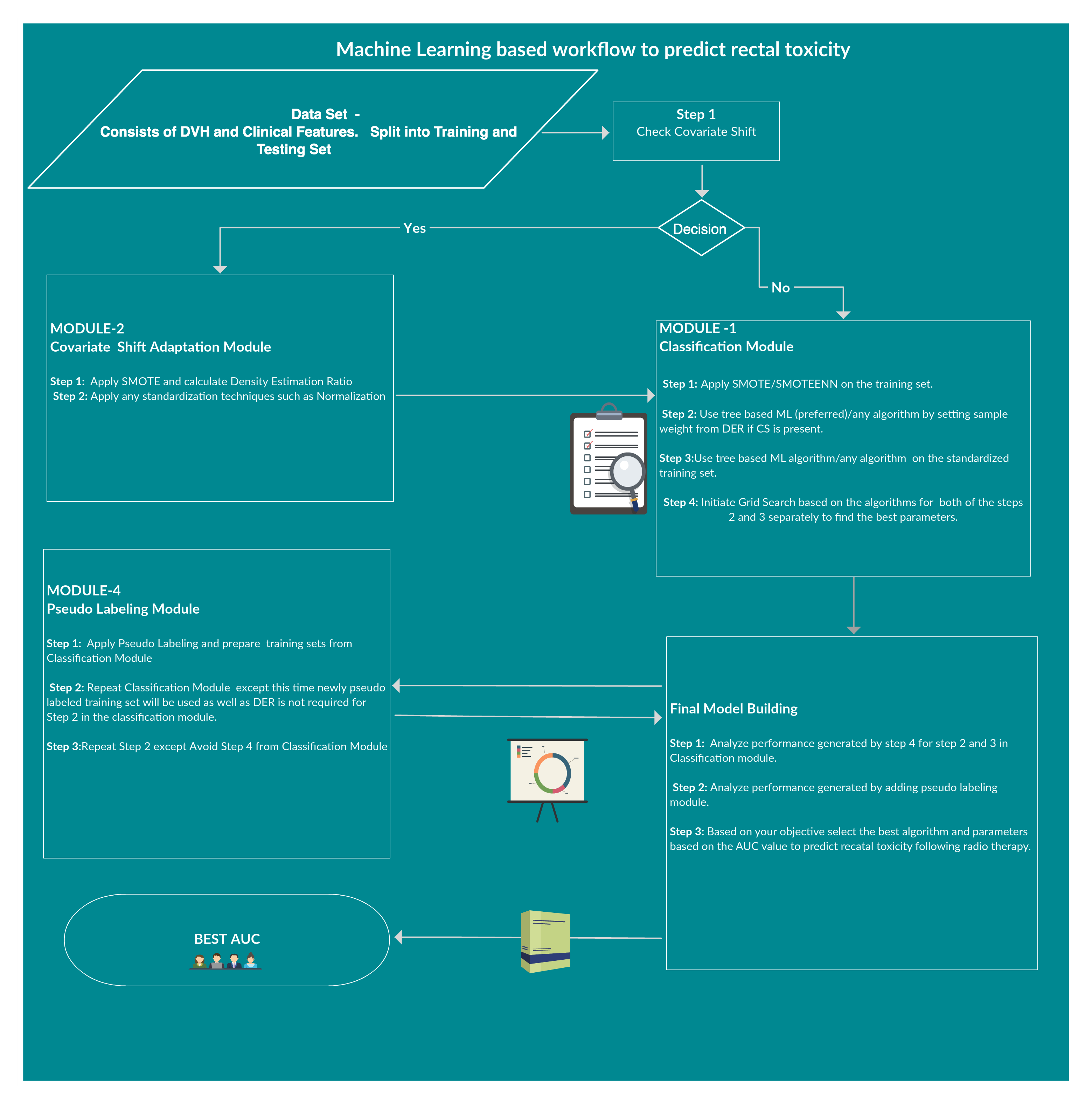
Rectal bleeding Toxicity prediction following radiotherapy requires the integration of many heterogeneous variables, including patient (clinical history, age, etc.) and treatment (DVH, treatment techniques, etc.) characteristics, into predictive models. As predictive models require a large amount of data, overfitting issues may occur, such as having too many parameters for the number of events. Improv- ing toxicity thus becomes a trade-off between including a large amount of data (gathering as much information as possible) and not too much to cause overfitting. Besides, databases from different centers contain a major issue which is called Covariate Shift.To overcome these issues, different machine learning and deep learning approaches have been done to find a better framework for prediction.At the end, a novel framework ‘Ramport Flow’ has been proposed and tested in several scenarios which performed better.
The records of 591 patients who underwent radiotherapy for localized prostate cancer were prospectively registered in three French academic institutions as part of three prospective studies: the STIC-IGRT study (clinical trial NCT00433706, accrual between June 2007 and November 2012; cut-off date: January 2015), the GETUG 14 study (clinical trial NCT00104741, accrual between December 2003 and August 2008; cut-off date: March 2016), and the BDD IMRT-cancer prostate ICM study (accrual between Febru- ary 2003 and October 2008; cut-off date: January 2014). Approval was granted for these studies by the appropriate ethics committee, and all patients provided informed consent in accordance with the latest Helsinki declaration. The target volume was defined as the prostate and seminal vesicles. The pelvic lymph nodes were not irradiated. The mean dose delivered to the prostate was 79.3 Gy (range: 76–80) at 2 Gy per fraction, with 46 Gy delivered to the seminal vesicles. Patients were placed in the supine position for all simulations and treatments. The target volume and organs at risk (bladder, rectum, and femoral heads) were delineated on CT slices according to French GETUG recommendations. The plan- ning target volume was calculated from a 10-mm margin all around the prostate and seminal vesicles, except in the posterior direction, where a 5-mm margin was applied. The rectal DVH com- plied with GETUG recommendations, namely fixing the volume receiving 72 Gy (V72) at < 25% and the maximum dose (within 1.8 cc) at <76 Gy.All patients were prospectively evaluated 2 months following radiotherapy, then every 6 months thereafter. Late rectal toxicity was defined as any event occurring over 6 months after beginning radiotherapy. Rectal toxicity was scored according to the common terminology criteria for adverse events (CTCAE), Version 3.0. Patients with a history of hemorrhoids were not included in the analysis. Mean follow-up was 67 months (range: 32–152). The 5- year risk of Grade 2 RB was 11% (95% CI: 8.4–13.6%), calculated using the Kaplan–Meier method.
The cohort was split into a training group, comprising 337 patients from two treat- ment centers, and a validation group of 254 patients treated in another center. The training group was used to train our models and the validation cohort was used for evaluating the RB-prediction capability of both the ‘‘standard” parameters and the original calculated features, using the Cox proportional hazards model generated during the training step. All the predictions were made in 3 years timeframe.
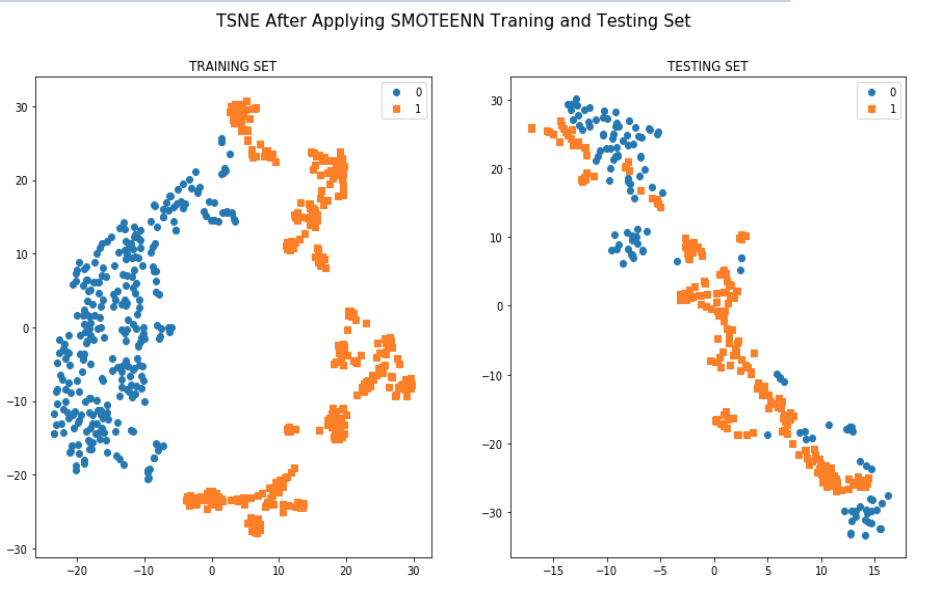
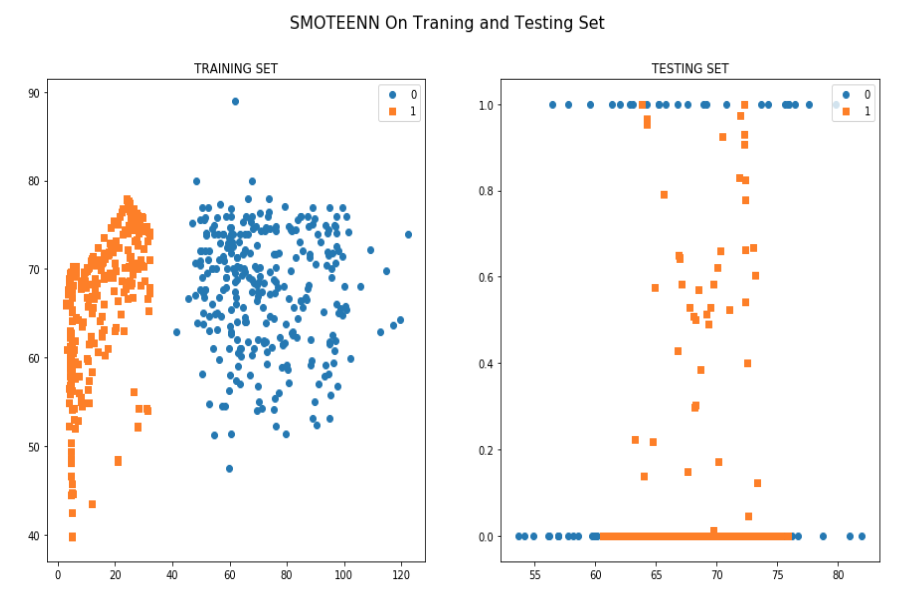
As the patients came from different data centers it has an issue of Covariate shift(training and validation cohort has different distribution) which was found both in the standard and manually calculated features list. The score was 0.94 which is high.This is a challenging task to overcome this problem in this small dataset.
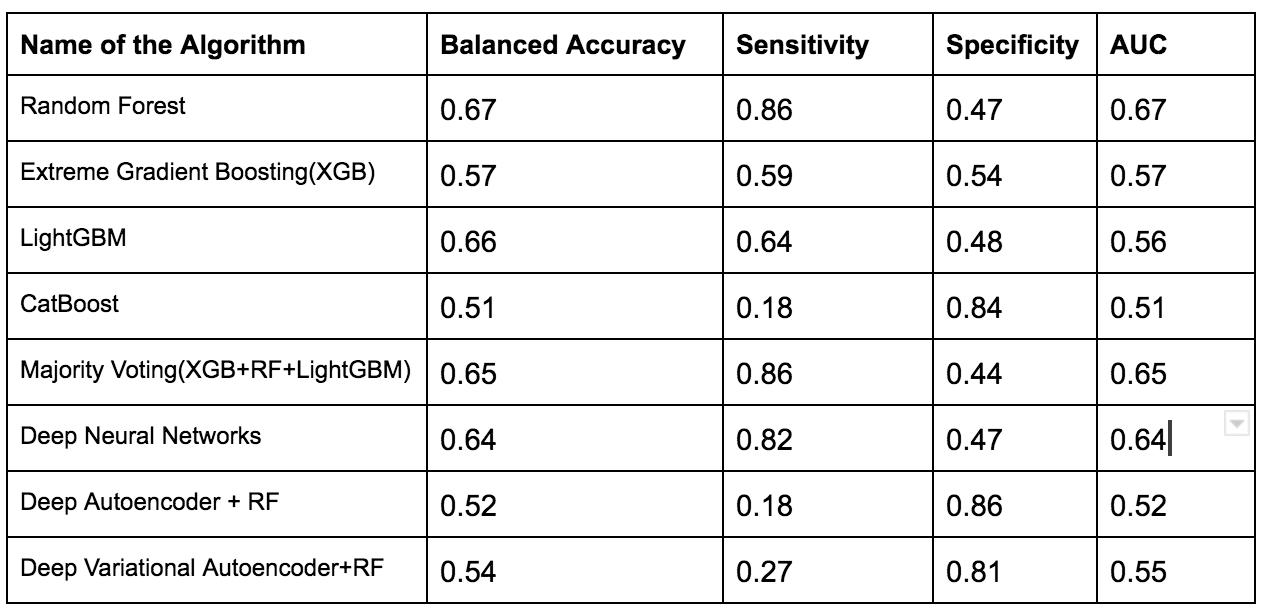
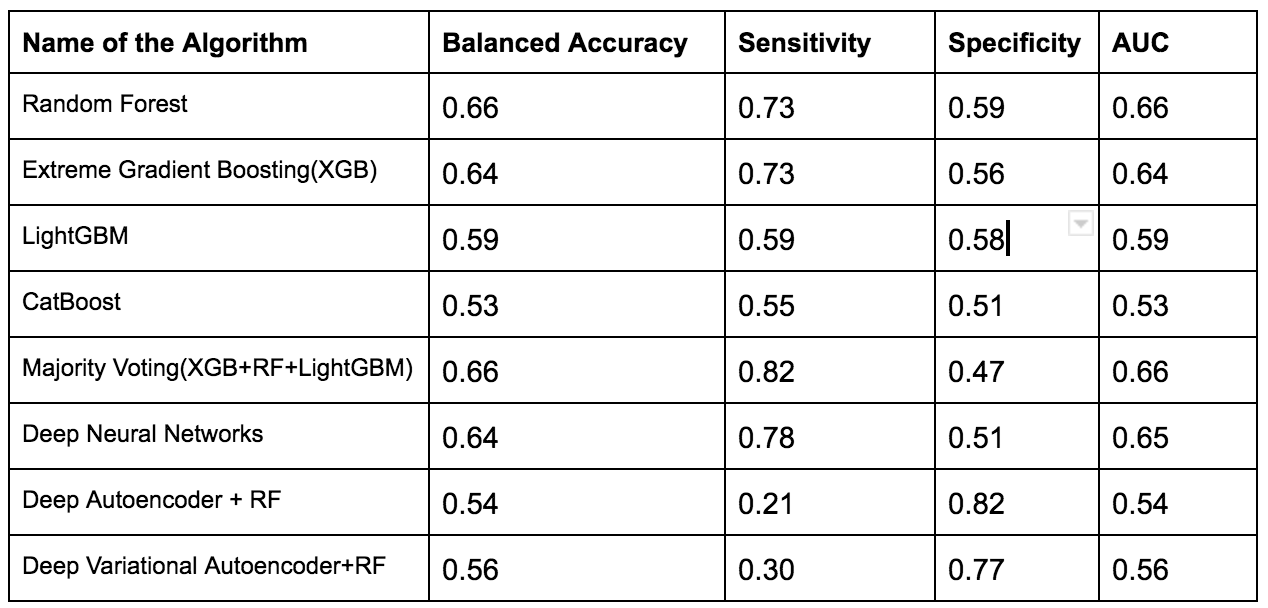
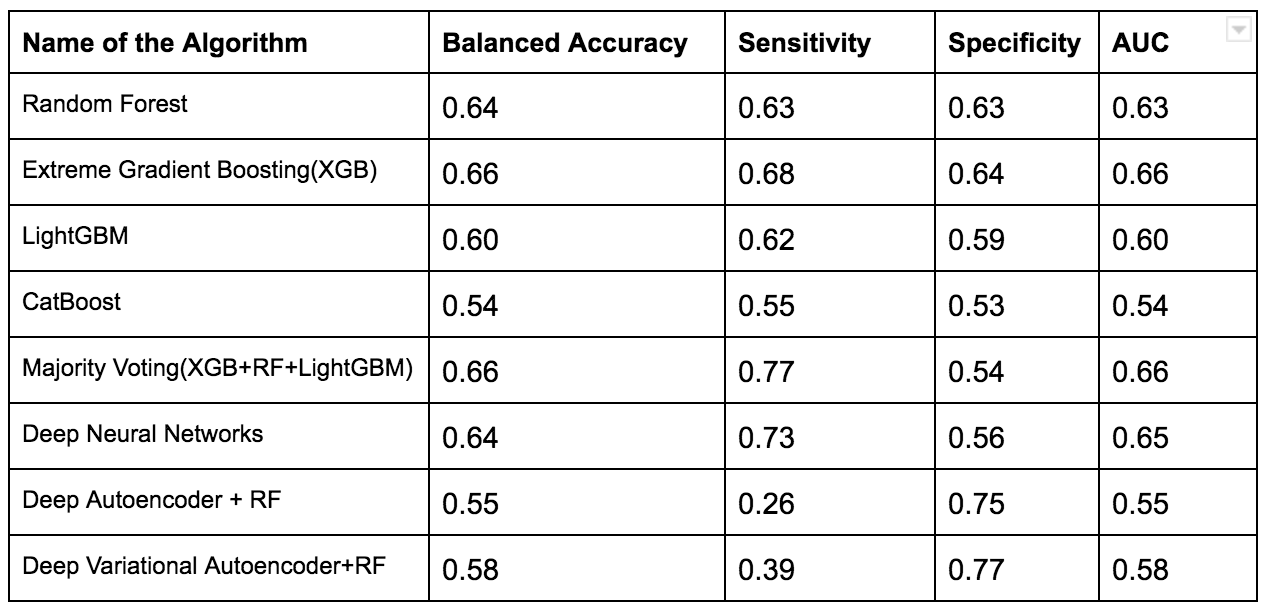
To overcome this issue several techniques such as Normalization,Min-Max Scaling,Weight Estimation using Density Estimation Ratio and ComBat Algorithms were evaluated too.After several iterations of using several machine learning algorithms(Random Forest,XGB,Catboost,LightGBM) and Deep Learning algorithms(Deep Neural Networks,Deep Autoencoder,Deep Variational Autoencoder), we were able to develop a framework which showed that if we use this novel approach it improves accuracy in many scenarios.In the below table, important results are shown.
Experiments with Adapting Covariate Shift Problem using Density Estimation Ratio (with proposed Framework)
We have used intuitive heuristics to provide feedback for the radiologists to improve the predictability of the framework which might help in any machine learning analysis too.
According to the two graphs that we generated it is apparent that training dataset from two centers are really significant in separating toxic patients from non toxic patients. All the approaches or machines used in these two centers can help to define a standard procedures for predicting rectal bleeding prediction following prostate cancer radiotherapy.
PET/CT images for stage II and III non-small cell lung cancer outcome prediction
Our goal was to design a deep convolutional neural network based workflow for non-small cell lung cancer (NSCLC) outcome fully automated prediction, i.e. without the need for tumor segmentation, as is usually required for radiomic analysis.
Several modified versions of 3D deep convolutional neural networks (CNN, e.g., C3D and Inception) were evaluated using computed tomography (CT) images as input.
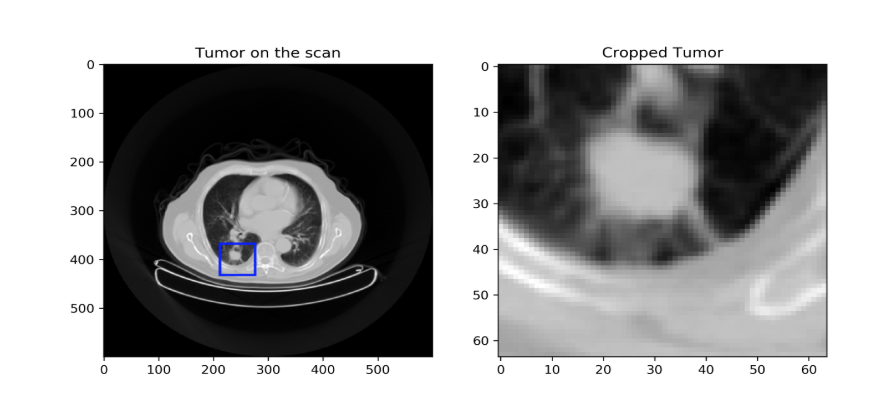
The proposed workflow is divided into two modules. The first consists of a primary tumor detector relying on a 3D CNN trained on two publicly available datasets of CT scans (1397 and 888 patients) with or without lung nodules/tumors (not necessarily NSCLC). This CNN was also trained to classify the detected lesion as normal or cancerous. This trained tumor detector was then evaluated in 3 different cohorts of patients with NSCLC (110, 422 and 61 patients). The goal is to feed only the image region containing the primary tumor to a second CNN trained specifically for outcome prediction. We assumed that training would be more efficient if the CNN can focus on the primary tumor only instead of the whole image. Two different methods were compared to design this second module: first, a modified version of the 3D CNN used in the first module (detector) with transfer learning using the tumor region as input. Second, we created 8×8 tiles where each tile has the size of 64×64 pixels as 2D input representing the entire 3D region containing the tumor as a training data and ability to classify patients below or above the median overall survival in the three NSCLC cohorts was evaluated.
The accuracy of the trained CNN for differentiating normal from cancerous nodule in the two lung CT scans datasets was 86%. It was able to correctly detect the primary tumor in 70% of patients, detected only partial areas of the tumor in 30% of patients and failed for the remaining 10% patients. The best accuracy for outcome prediction for patients with tumor correctly detected by the first module was 70% using 2D networks.
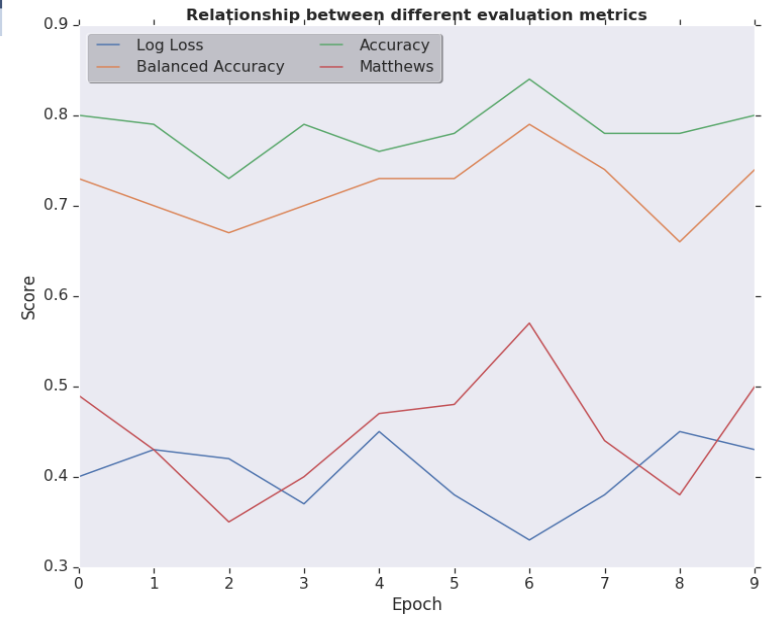
Relationship Between Different Evaluation Metrics for the model which is capable of separating normal from cancerous nodule
The availability of large datasets is usually a crucial requirement for efficient training of CNN. Our work shows that by relying on a two-step approach these requirements can be in part alleviated.Further experiments are ongoing including new cohorts from LITSI(15 Patients) and LaTIM(28 Patients) to improve the accuracy of the prediction as well the tumor detection with the addition of the PET component in case of PET/CT scans.
List of publications:
International journal papers
- Acosta O, Mylona E, Le Dain M, Voisin C, Lizee T, Rigaud B, Lafond C, Gnep K, de Crevoisier R. Multi-atlas-based segmentation of prostatic urethra from planning CT imaging to quantify dose distribution in prostate cancer radiotherapy. Radiother. Oncol. 2017;125:492–499.
- Mylona E, Acosta O, Lizee T, Lafond C, Crehange G, Magné N, Chiavassa S, Supiot S, Ospina JD, Campillo-Gimenez B, Castelli J, de Crevoisier R. Voxel-Based Analysis for Identification of Urethrovesical Subregions Predicting Urinary Toxicity After Prostate Cancer Radiation Therapy. Int J Radiat Oncol Biol Phys, 2019. 104 (2): 343-54.
- Largent A, Barateau A, Nunes JC, Mylona E, Castelli J, Lafond C, Greer P, Dowling J, Baxter J, Saint-Jalmes H, Acosta O, de Crevoisier R. Comparison of deep learning-based and patch-based methods for pseudo-CT generation in MRI-based prostate dose planning. Int J Radiat Oncol 2019. doi:10.1016/J.IJROBP.2019.08.049.
- Mylona E, Cicchetti A, Rancati T, Palorini F, Fiorino C, Supiot S, Magne N, Creange G, Valdagni R, Acosta O, de Crevoisier R. Local dose analysis to predict acute and late urinary toxicities after prostate cancer radiotherapy : assessment of cohort and method effects. Radiother. Oncol. (Submitted)
Peer-reviewed conference papers
- Mylona E, Lebreton C, Fontaine C, Crehange G, Magné N, Supiot S, de Crevoisier R, Acosta O. Comparison of machine learning algorithms and oversampling techniques for urinary toxicity prediction after prostate cancer radiotherapy. IEEE proceedings (Accepted, in BIBE 2019)
Communications in international conferences
- Lizee T, Mylona E, Lafond C, Supiot S, Acosta O, de Crevoisier R. Analysis of the urethro-vesical region for urinary toxicity prediction after prostate radiotherapy. Radiother. Oncol. 2018;127:432-433. ( in ESTRO as poster)
- Mylona E, Acosta O, Lizee T, Lafond C, Crehange G, Magné N, Chiavassa S, Supiot S, Ospina JD, Campillo-Gimenez B, Castelli J, de Crevoisier R. Bladder and urethra subregions predicting urinary toxicity after prostate cancer radiotherapy. Radiother. Oncol. 2019;133:449. (in ESTRO as poster)
- Mylona E, Cicchetti A, Rancati T, Palorini F, Supiot S, Magne N, Creange G, Acosta O, de Crevoisier R. Predicting urinary toxicity via 2D and 3D dose map analyses in prostate cancer radiotherapy. Radiother. Oncol. 2019;133:326. (in ESTRO as oral communication)
- MD. Ibrahim, E. Mylona, N. Boussion, O. Acosta, R. De Crevoisier, M. Hatt ,Machine learning methods to predict rectal bleeding after prostate cancer radiotherapy, ESTRO-2020(Submitted)
- MD Ibrahim, J. Castelli, C. Cheze Le Rest, O. Acosta, D. Visvikis, R. De Crevoisier, M. Hatt,Deep CNN on PET/CT images for NSCLC automated tumor detection and outcome prediction, ESTRO-2020(Submitted)
- MD. Ibrahim, D. Visvikis, C. Cheze Le Rest, M. Hatt, Relying on deep convolutional neural networks on PET/CT images for stage II and III non-small cell lung cancer outcome prediction, Annual congress of the EANM, 2019
- MD. Ibrahim, D. Visvikis, C. Cheze Le Rest, M. Hatt, A 3D Deep Convolutional Neural Network for Lung Cancer Survival Prediction Using Transfer Learning, AAPM annual meeting, 2018
Communications in national conferences
- Lizée T, Acosta O, Mylona E, Le Dain M, Lafond C, Riet FG, de Crevoisier. Développement d’une méthode de segmentation automatique de l’urètre sur tomodensitométrie de planification permettant d’évaluer la dose urétrale en cas de radiothérapie prostatique. Cancer/Radiothérapie 2017;21:706. doi:10.1016/J.CANRAD.2017.08.062.
- Acosta O, Mylona E, Lafond C, Créhange G, Supiot S, Castelli J, de Crevoisier R. Identification de sous-régions rectale et urétrovésicales hautement prédictives de toxicité en cas d’irradiation prostatique. Cancer/Radiothérapie 2019;23:791. doi:10.1016/J.CANRAD.2019.07.010.
Communications in workshop
- MD. Ibrahim, D. Visvikis, C. Cheze Le Rest, M. Hatt, 3D Deep Convolutional Network in Lung Cancer Classification, “Prediction and Modeling of response to Molecular and External Beam Radiotherapies” workshop ,Manoir de Kerdréan, Le Bono, France, September 20th- 23rd, 2017
- MD. Ibrahim, D. Visvikis, C. Cheze Le Rest, M. Hatt,Designing a Workflow in Medical Imaging using Transfer Learning,13th IEEE-EMBS International Summer School on Biomedical Imaging,Saint-Jacut de la Mer, Emerald Coast, Brittany, France,14-22 June 2018
Collaboration / Mobilities:
Within the project:
LTSI – LATIM : Data sharing for toxicity prediction using deep learning
LTSI-LATIM : Data sharing for stage II and III non-small cell lung caner outcome prediction
National:
LTSI – Nantes (Stephane Supiot) and LTSI-Dijon (Gilles Creange) for data collection for urinary toxicity analysis: resulted in multicentric study of 272 patients.
International:
LTSI- Italy, Fondazione IRCCS Istituto Nazionale dei Tumori (Tiziana Rancati): Urinary toxicity prediction using dose-surface maps. Periode:October 2018
LTSI- Australia, University of Western Australia (Martin Ebert) : External validation of dose-volume map models. Periode: December 2018-April 2019.