By combining the two approaches of epileptic and tumor patients, we expect to:
-Convert the current proof-of-concept of intraoperative EP recordings in a standardized, reliable and reproducible method, allowing real time and in-vivo measurement of local electrophysiological state (e.g. excitability) and structural connectivity.
– Provide a detailed characterization of each type of EP (global shape (P0, N1, N2, …), delay, amplitude, peak width), and correlate these characteristics with other clinical data, in order to guide the surgery,
-Develop optimized parameters (selectivity, reduction of injected charges, etc.) and new stimulation paradigms for electrical stimulation used in anatomo-functional mapping.
-Development of the imaging of healthy and diseased tissues by electrophysiology and modification of evoked potentials (e.g. probe the degree of connections through the attenuation of the amplitude of CCEP).
– Model the effects of electrical stimulation and its diffusion, mainly from axon models.
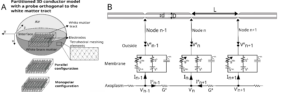
Figure A Partitioned 3D conductor model. The current injected in the 3D model at the level of the electrode contacts imposes an electric potential field. Here these electrodes are represented on the tract, in order, from top to bottom: orthogonal and parallel to the tract (meaning orthogonal or parallel to the z axis) then monopolar. The definition of this potential field is done by a finite element method: a tetrahedral mesh is created where on each tetrahedrons summit is calculated the electric potential in quasi-static condition.
Figure B: Electrical circuit of the dynamic membrane model and the cable equation. The central node #n gives the description of the currents in play for the resolution of membrane potential. The bottom line describes the contribution of the axoplasmic currents to this central node.
More particularly, we plan to embark on modeling the effects of DES on the cortex in the context of the Inria French-Japan associate team we have with Pr Matsumoto. We aimed at identifying the best stimulation parameters (e.g. current strength, inter-electrode distance, pulse-width, etc) that provoke direct trigerring of action potentials in the white matter path- ways (without any need for integration processes at the level of the grey matter). As we do not have expertise in modeling dendritic or somatic compartments at the level of pyramidal neurons, we are thinking about simplifying the biophysics of these compartments and are thinking about possible collaborations which are not yet cleary identified.
Last but not least, technological Transfer is possible, especially with companies that provide Neurosurgery services stimulator for intraoperative functional mapping. Transfer focus on the development of new modes of stimulation with a different setting. This objective is currently being discussed with Innopsys which develop intra-operative electrode and stimulator and Neurinnov which develops advanced stimulator both for intra-operative and for implants.