Neural Coding: Identification of cortical networks from high-resolution EEG: application of the mental information therory
1.General context
The concept of brain connectivity is at the heart of many studies in neurosciences, either in the field of cognitive (Stevens, 2009) or clinical (Uhlhaas and Singer, 2006) research. The leading idea is that networks of interconnected distinct brain regions underlie normal brain functions (involved in learning, memory, behavior adaptation to stimuli, emotions) as well as pathological processes in some brain disorders like epilepsy, autism or schizophrenia. The concept of brain connectivity has long been a topic of tremendous interest for neuroanatomists. Seminal studies were performed at the beginning of the last century by S. Ramón y Cajal (Ramón y Cajal, 1894). Before Cajal’s discoveries, very little was known about the neuronal elements of the nervous system, and the connections between its different parts were purely speculative. Since then, several attempts to formalize this concept have followed. It is now well established that the term “brain connectivity” refers to a model of anatomical links (“anatomical” or “structural” connectivity”), statistical dependencies (“functional connectivity”) or causal interactions (“effective connectivity”) between sites located in different brain areas within the central nervous system (Sporns, 2010). Over the recent past years, one major challenge in neuroscience is to identify these networks from data provided by available neuroimaging techniques, either structural (diffusion tensor imaging or DTI) or functional (electroencephalography or EEG, magnetoencephalography or MEG, functional magnetic resonance imaging or fMRI). Solutions to this difficult problem would significantly advance our understanding of normal brain functions like memory, but also of some brain disorders in the prospect of discovering possible therapies (review in (Horovitz and Horwitz, 2012)).
2.Objective and contribution of research teams
Objective. In this context, we want to go beyond the state-of-the-art in brain connectivity estimation. The proposed research project focuses on the identification of brain circuits from real EEG data recorded with a high spatial resolution, either in healthy subjects (according to neuropsychological protocols involving memory-related tasks), or in patients with epilepsy (experiencing repetitive transient abnormal activity during non-ictal periods). The objective is to combine some of the above-mentioned methods for characterizing the functional brain connectivity with a novel theory of information processing in the brain.
LTSI contribution. In September 2011, the SESAME team (head Fabrice Wendling) of LTSI was awarded by the French Foundation for Epilepsy Research (FFRE) for the financing and installation of 256-channel EEG data acquisition system (so called high-resolution EEG). This equipment is installed at the Rennes University Hosptial in the Neurology Department which becomes the first unit in France using such a system. Installed in January 2012, the system can be used both for cognitive research and clinics (epilepsy). The unique feature of this system is the “almost full” coverage of the subject’s head by surface electrodes (including the face and the temporo-basal regions). As an example, the classical EEG makes use of 19 to 32 electrodes only. The increased number of electrodes will allow us to improve the performance of source localization methods (inverse problem). In addition, we expect to get a much finer (i.e. local) reflection of the overall activity of the neocortex as the increased number of electrodes also offers the possibility of spatially filter EEG signals using Laplacian montages. Moreover, the temporal resolution of the recorded signals is excellent (in the order of 1 ms). From such signals, it is possible to estimate the functional connectivity and many methods have been proposed (Wendling et al., 2009; Wendling et al., 2011). However, up to now, none of them has proven to be uniformly effective, each one being sensitive to a change in the relational model (Ansari-Asl et al., 2006).
Lab-STICC contribution. Recently, an original theory has been developed in the team CACS/IAS (algorithm-silicon interaction) of Lab-STICC with the aim of designing new neuro-inspired intelligent machines. This theory is referred to as the “mental information theory” ((Berrou 2011), (Gripon and Berrou, 2011), see also Vincent Grippon’s thesis, 2011). This has been recently introduced by Claude Berrou, mainly known as being one of the co-inventors of turbo codes. Based on distributed error correcting codes and graph theory, this theory has attracted the interest of the European Research Council (ERC) which has awarded Claude Berrou an ERC advanced grant in 2011. This theory might prove highly relevant in the extraction of “sub-graphs” from a high-dimensional graph obtained from the EEG and describing the relatively local activity of the cerebral cortex in human. As completely original in the field of neuroscience, it would challenge the already-proposed methods mentioned above. Briefly, this theory considers all cortical networks as an informational model of recurrent graphs where each node is not a single neuron, but a group of neurons, called a microcolumn. These microcolumns (or “fanals” according to the vocabulary of this theory) can merge to form columns (or clusters) that in turn, form macrocolumns together. In this model, mental pieces of information, called “infons”, are carried by cliques, that is, small aggregates of microcolumns that activate together. The clique can be viewed as a subgraph in the vast network of microcolumns present in the human neocortex (Gripon, 2011). By introducing the properties of redundant coding and parcimony in the recurrent graph model, C. Berrou and his team showed theoretically how the cliques are forming during a learning process corresponding to the encoding of chunks of information (or infons) that progressively arrive to the brain.
3.Added value and repercussions
It is worth noting than high-resolution EEG signals can offer the unique opportunity to get closer to the activity of macrocolumns located in the neocortex. Therefore, we are in a situation where we can test and validate some aspects of the mental information theory on non-invasive data recorded in healthy subjects. We will define, with the help of expert neuropsyschologist colleagues, appropraite tasks related to learning/working memory and involving the activation of neocortical circuits. Though the use of high resolution EEG signals, the addressed identification problem (subgraphs in a graph containing more than 250 nodes) is consistent in terms of complexity. Additional information available at each node (like brain rhythms in the theta or gamma frequency bands) can also complement connectivity measures and thus increase the complexity.
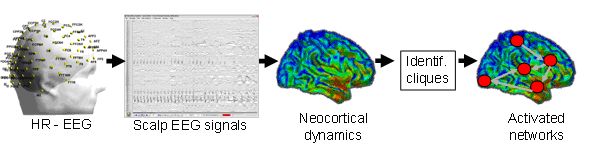 Thus, the project can lead to strong added-value in terms of innovation in the field of brain connectivity by combining the efforts of two research teams (Lab-STICC and LTSI) of the Labex CominLabs. The impact would be major in terms of advancing our knowledge of brain connectivity during cognitive activity in particular, and of brain information, processing in general. The methods developed in the team of C. Berrou are highly innovative and their application to the EEG would be a first in the field.
Thus, the project can lead to strong added-value in terms of innovation in the field of brain connectivity by combining the efforts of two research teams (Lab-STICC and LTSI) of the Labex CominLabs. The impact would be major in terms of advancing our knowledge of brain connectivity during cognitive activity in particular, and of brain information, processing in general. The methods developed in the team of C. Berrou are highly innovative and their application to the EEG would be a first in the field.
References:
- Ansari-Asl K, Senhadji L, Bellanger JJ, Wendling F. 2006. Quantitative evaluation of linear and nonlinear methods characterizing interdependencies between brain signals. Phys Rev E Stat Nonlin Soft Matter Phys 74(3 Pt 1):031916.
- Arnhold J, Grassberger P, Lehnertz K, Elger CE. 1999. A robust method for detecting interdependences: application to intracranially recorded EEG. Physica D: Nonlinear Phenomena 134(4):419-430.
- Bhattacharya J. 2001. Reduced degree of long-range phase synchrony in pathological human brain. Acta Neurobiol Exp (Wars) 61(4):309-18.
- Franaszczuk PJ, Bergey GK. 1999. An autoregressive method for the measurement of synchronization of interictal and ictal EEG signals. Biol Cybern 81(1):3-9.
- Gotman J. 1983. Measurement of small time differences between EEG channels: method and application to epileptic seizure propagation. Electroencephalogr Clin Neurophysiol 56(5):501-14.
- Gourevitch B, Bouquin-Jeannes RL, Faucon G. 2006. Linear and nonlinear causality between signals: methods, examples and neurophysiological applications. Biol Cybern 95(4):349-69.
- Gripon V. 2011. Networks of Neural Cliques. Thèse de doctorat. Telecom Bretagne. Mention: Sciences et Techniques de l’Information et des Communications:121 pages
- Gripon V, Berrou C. 2011. Sparse neural networks with large learning diversity. IEEE Trans Neural Netw 22(7):1087-96.
- Horovitz SG, Horwitz B. 2012. Introduction to research topic – brain connectivity analysis: investigating brain disorders. Part 2: original research articles. Front Syst Neurosci 6:4.
- Kiebel SJ, Garrido MI, Moran R, Chen CC, Friston KJ. 2009. Dynamic causal modeling for EEG and MEG. Hum Brain Mapp 30(6):1866-76.
- Pikovsky A, Rosenblum M, Kurths J. 2001. Synchronization : a universal concept in nonlinear sciences. Cambridge: Cambridge University Press.
- Ramón y Cajal S. 1894. Les nouvelles idées sur la structure du système nerveux chez l’homme et chez les vertébrés. L. Azoulay, editor. C. Reinwald.
- Rosenblum M, Pikovsky A, Kurths J. 2004. Synchronization approach to analysis of biological signals. Fluctuation Noise Lett. 4:L53-L62.
- Sporns O. 2010. Networks of the Brain. MIT Press.
- Stam CJ, van Dijk BW. 2002. Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Physica D: Nonlinear Phenomena 163(3-4):236-251.
- Stevens MC. 2009. The developmental cognitive neuroscience of functional connectivity. Brain Cogn 70(1):1-12.
- Uhlhaas PJ, Singer W. 2006. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52(1):155-68.
- Wendling F, Ansari-Asl K, Bartolomei F, Senhadji L. 2009. From EEG signals to brain connectivity: a model-based evaluation of interdependence measures. J Neurosci Methods 183(1):9-18.
- Wendling F, Chauvel P, Biraben A, Bartolomei F. 2011. From intracerebral EEG signals to brain connectivity: identification of epileptogenic networks in partial epilepsy. Front Syst Neurosci 4:154.

