Lower extremity peripheral artery disease (LEPAD) is a highly prevalent chronic disease that affects ~800 000 patients in France. Despite LEPAD patients have a high risk of death from cardiovascular causes (Criqui et al. 1992), LEPAD is underdiagnosed, undertreated (Blacher et al. 2006) and public knowledge of the disease is poor (Hirsch et al. 2007). Most patients are asymptomatic and the prevalence of asymptomatic LEPAD is between 10 to 20% for subjects older than 55 years, according to the French health authority (“Haute Autorité de Santé”, 2006). Both symptomatic and asymptomatic LEPAD patients are at high risk of adverse cardiovascular outcomes, and the 5-year mortality rate from cardiovascular causes is between 18 and 30% (French health authority, 2006). LEPAD is caused primarily by atherosclerosis that induces an insufficient blood flow to the tissues (named ischemia) due to the narrowing of the arteries.
Assessment of lowers limbs function is an important step in the management of LEPAD patients. This enables the clinician to objectively quantify the impact of ischemia on the functional capacity of the patients. Lowers limbs function is a powerful predictor of mortality and mobility loss (Guralnik et al., 1995). This is also an outcome measure used to assess the effects of therapeutic interventions (Fakhry et al., 2012). One of the most used metrics is the evaluation of the walking capacity through the analysis of the maximal walking distance (MWD). MWD on a treadmill is currently the “gold standard” method used to measure MWD in LEPAD patients. However, treadmill evaluation of MWD remains protocol dependent, technically demanding, time-consuming, and limited to vascular laboratories. Furthermore, treadmill MWD may not be correlated with the “true” free-living MWD at usual pace and on a flat surface and does not correlate with the patient’s perception of disability. Beyond the sole issue of MWD assessment, supervised walking exercise is an efficient therapeutic strategy to improve the classic symptom associated with LEPAD (intermittent claudication) and thus to improve walking capacity (Fakhry et al., 2012). Unfortunately, supervised walking exercise is often not prescribed because of treatment cost, transportation difficulties and lack of insurance reimbursement. Community-based walking programs could be an attractive alternative approach, but an important methodological issue is to adequately control and quantify the total exercise dose of the program.
Ambulatory assessment of walking capacity in LEPAD patients could overcome most of theses limitations. A new way to assess community walking capacity in LEPAD patients has been proposed using GPS technology (Abraham et al., 2012 ; Le Faucheur et al., 2007, 2008, 2010 ; Noury-Desvaux et al., 2012). This method opens new perspectives for walking capacity assessment but also to conduct community-based walking programs. However, technical feasibility of the technique has to be improved to extent the clinical value and the diffusion of the technique.
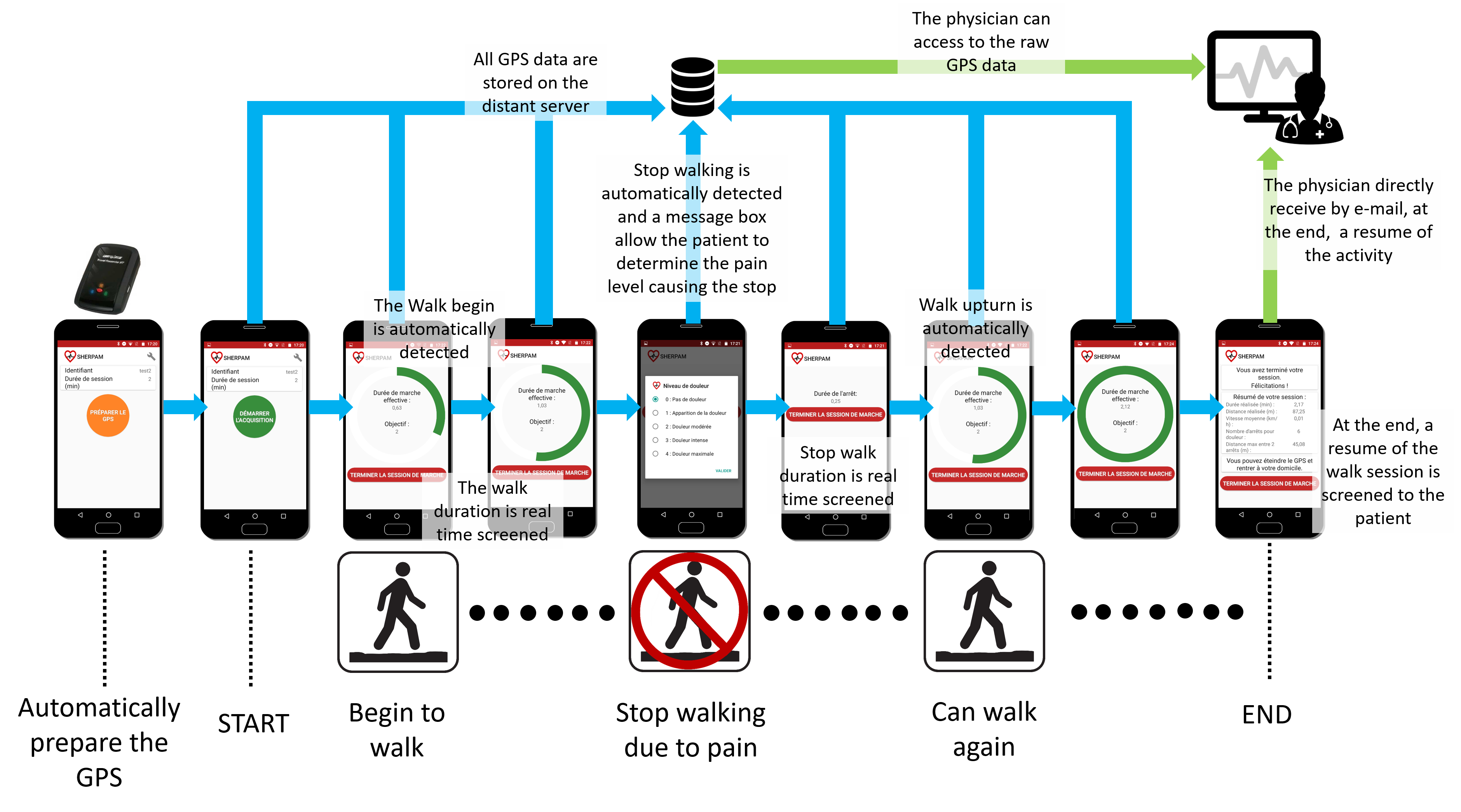
One goal of the SHERPAM project is to design a system that would enable the clinicican to have a continuous ambulatory measurement of the walking pattern of LEPAD patients during outdoor walking sessions. This system would help the clinician to speciffically assess the walking limitations induced by the ischaemic pain in free-living conditions. Thanks to the SHERPAM project, a specific solution for LEPAD patients has been designed. This solution is based on an external GPS device (BT-Q1000XT, QStarz International Co., Taiwan) that communicates through a Bluetooth link with a Smartphone application. The application is automatically configured to communicate with the GPS receiver and all raw GPS data are continuously sent to a distant server through the Smartphone’ application. When a patient begins an outdoor walking session, the patient’ walk is automatically detected by the application and screened regarding the session target, for instance a pre-defined effective walking duration that should be reached (green progress circle on the figure below). If the patient stops his/her walk due to a walking pain, a message box appears allowing the patient to rank the pain level reached before to stop. This pain level is sent to the distant server and the stop duration is screened. Then, when the patient resumes his/her walk, the new walking period is automatically detected and screened. When the patient has ended his/her walk session, a brief summary with the main metrics regarding the walking pattern of the patient (walking distances and speeds, stops duration, pain intensity level reached,…) is sent by e-mail to the clinician. Furthermore, the latter can have an access to the GPS raw data, if needed.